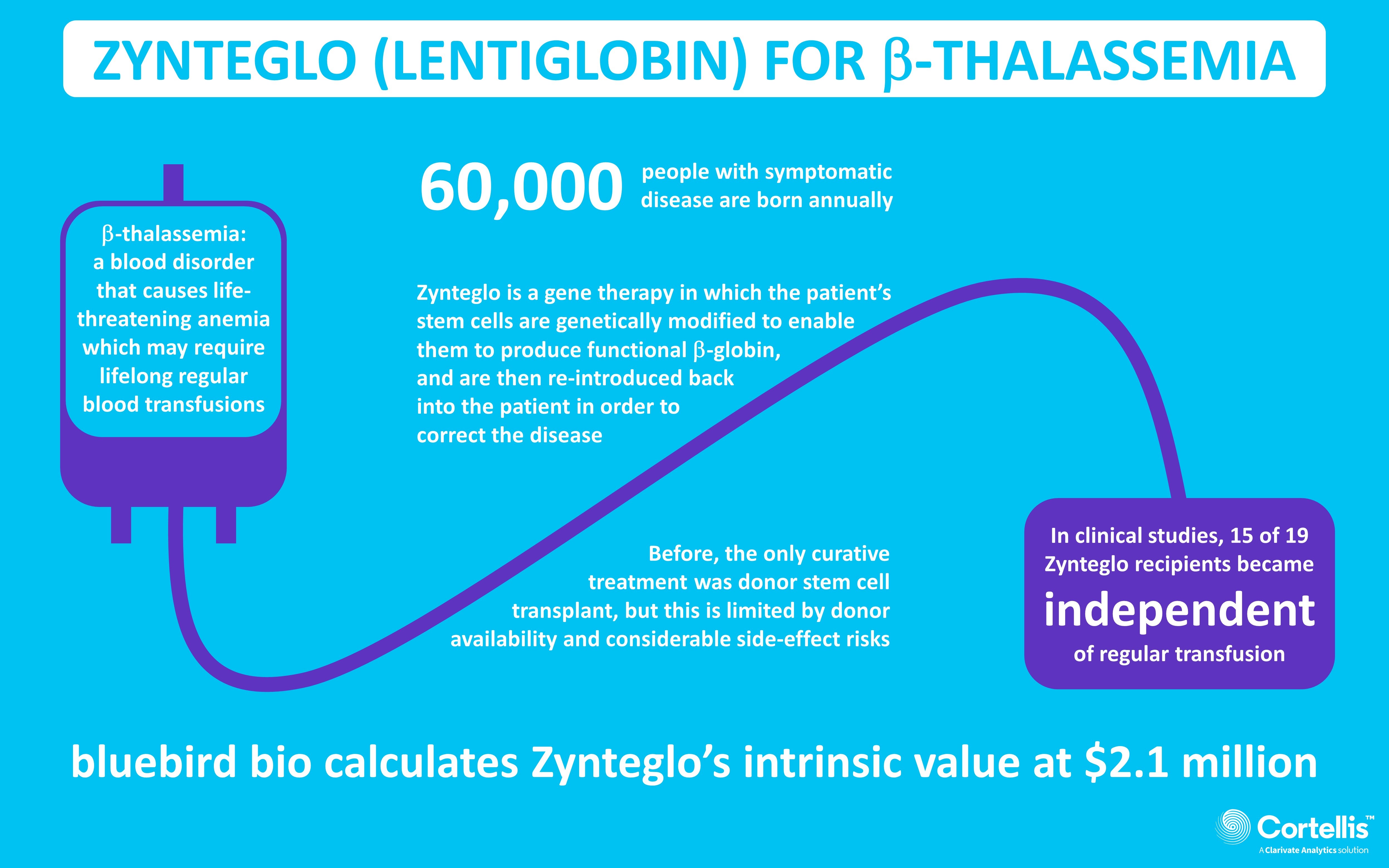
bluebird bio Announces FDA Approval of ZYNTEGLO®, the First Gene Therapy for People with Beta-Thalassemia Who Require Regular Red Blood Cell Transfusions | Business Wire
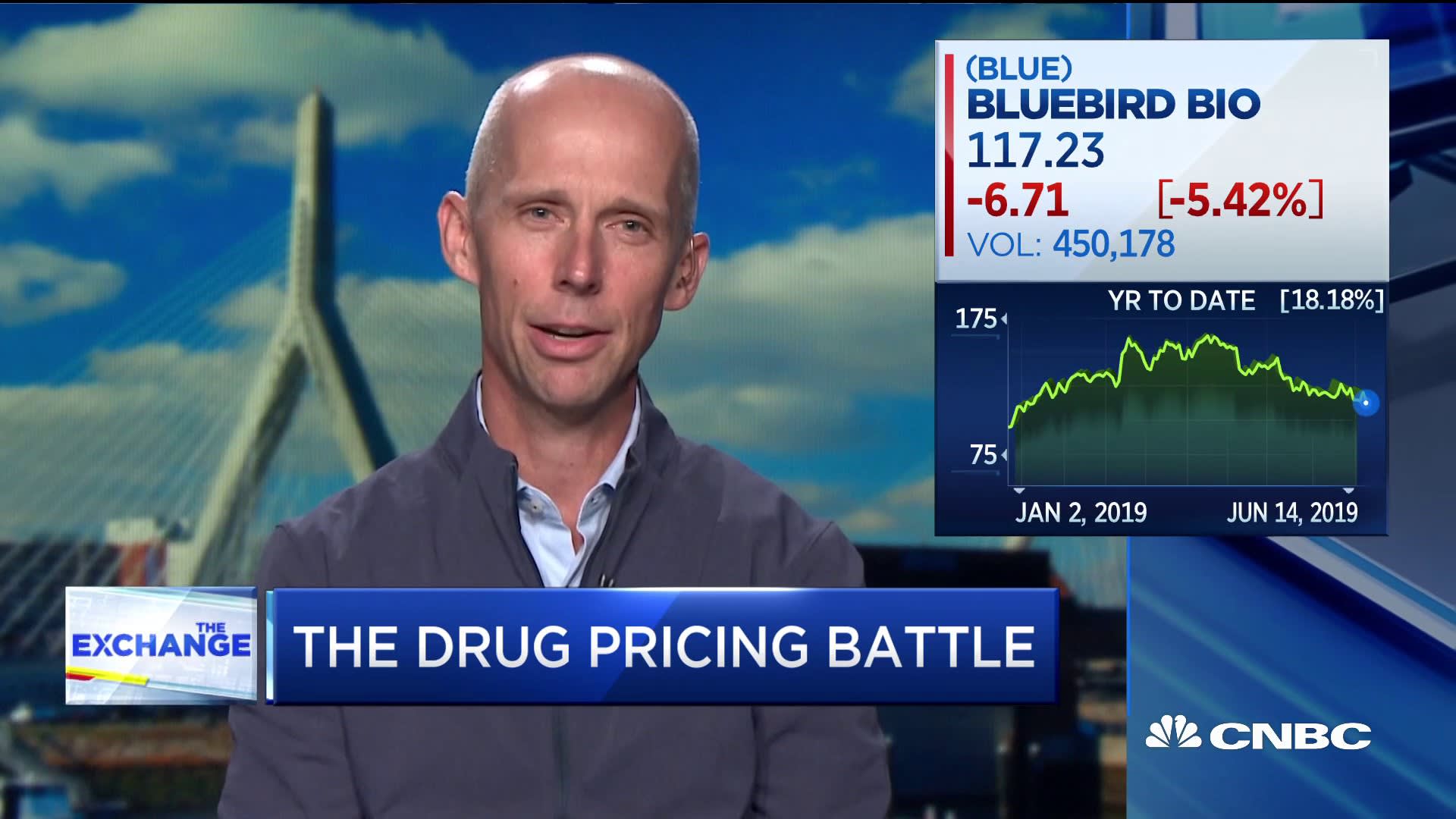
Bluebird bio's gene therapy Zynteglo could have high price tag defense in beta thalassemia - Pharmaceutical Technology

FDA Approves bluebird bio's ZYNTEGLO®, the First Gene Therapy for People with Transfusion-Dependent Beta-Thalassemia - The Cooley's Anemia Foundation

bluebird bio wins back-to-back landmark FDA approvals for first-in-class gene therapies - Pharmaceutical Technology

Somerville gene therapy firm bluebird bio nabs win with first FDA approval following split - Boston Business Journal

bluebird bio Announces FDA Approval of ZYNTEGLO®, the First Gene Therapy for People with Beta-Thalassemia Who Require Regular Red Blood Cell Transfusions - bluebird bio, Inc.

Bluebird Bio To Resume Zynteglo Gene Therapy Marketing In Europe After Positive Recommendation From PRAC