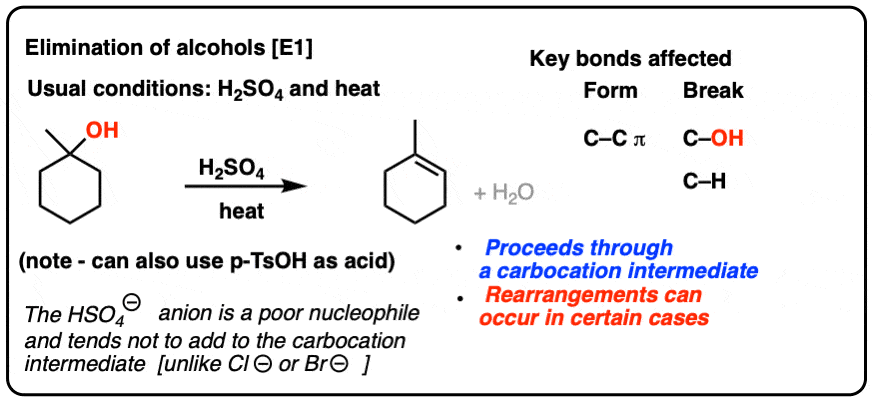
![SOLVED: 42 , The best synthesis of CH; NOz would be: A) Benzene HNO3 2 CH,Cl product H2 504 AlClj HNO3 CH;Cl Toluene product H2SO4 AlCl] HNO3 p-Xylene H2SO4 CH3Cl @-Nitrotoluene AlCl3 SOLVED: 42 , The best synthesis of CH; NOz would be: A) Benzene HNO3 2 CH,Cl product H2 504 AlClj HNO3 CH;Cl Toluene product H2SO4 AlCl] HNO3 p-Xylene H2SO4 CH3Cl @-Nitrotoluene AlCl3](https://cdn.numerade.com/ask_images/87e07e511eb7463e8ffded737a3ffc95.jpg)
SOLVED: 42 , The best synthesis of CH; NOz would be: A) Benzene HNO3 2 CH,Cl product H2 504 AlClj HNO3 CH;Cl Toluene product H2SO4 AlCl] HNO3 p-Xylene H2SO4 CH3Cl @-Nitrotoluene AlCl3

A carbon compound 'P' on heating with excess conc. H2SO4 forms another carbon compound 'Q' which on addition of hydrogen in the presence of nickel catalyst forms a saturated carbon compound 'R'. -
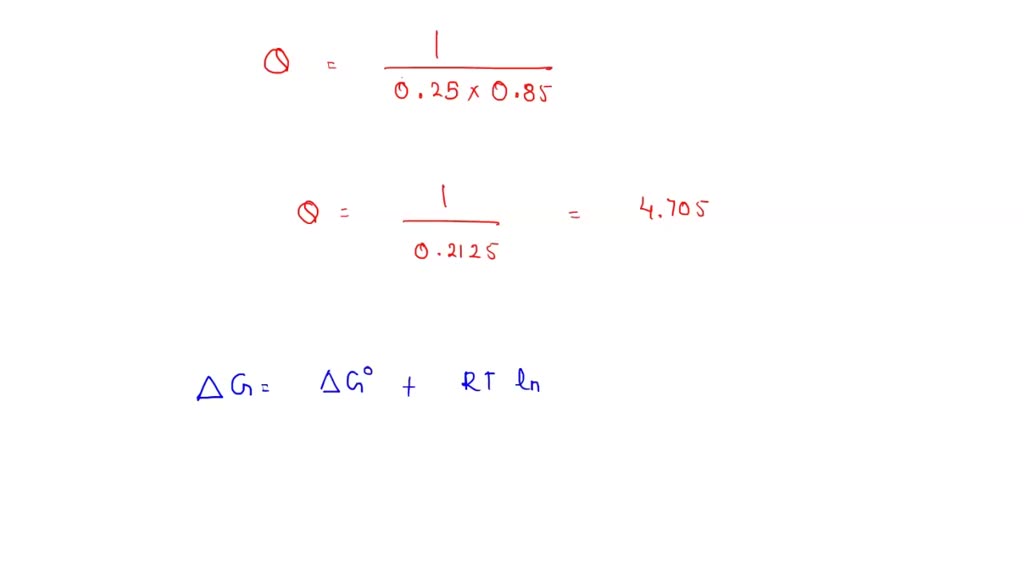
SOLVED: Calculate ΔGrxn at 298 K under the conditions shown below for the following reaction. SO3(g) + H2O(g) → H2SO4(l) ΔG°= -90.5 kJ P(SO3) = 0.25 atm, P(H2O) = 0.85 atm +86.7

Spectroscopic Measurement of pH in Aqueous Sulfuric Acid and Ammonia from Sub- to Supercritical Conditions | Industrial & Engineering Chemistry Research

What is the structure of the precipitate that forms after sulfuric acid has been added to the mixture of p-aminobenzoic acid in ethanol? Why do you need to add more acid even
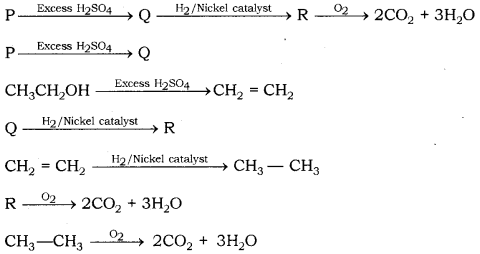
A carbon compound 'P' on heating with excess cone. H2SO4 forms another carbon compound - CBSE Class 10 Science - Learn CBSE Forum

1.) What is the structure of the precipiatate that forms after sulfuric acid has been added to the mixture of p-aminobenzoic acid in ethanol? Why do you need to add more acid
How much quantity of 98% sulphuric should be added to 48% sulphuric to make its percentage to 65%? - Quora

Number of `sigma` and `pi` bonds present in sulphuric acid molecule is ____ and ____ respectively. - YouTube
![p-Anisaldehyde (contains Acetic Acid, H2SO4) Ethanol Solution [for TLC Stain], TCI America, Quantity: 100 mL | Fisher Scientific p-Anisaldehyde (contains Acetic Acid, H2SO4) Ethanol Solution [for TLC Stain], TCI America, Quantity: 100 mL | Fisher Scientific](https://assets.fishersci.com/TFS-Assets/CCG/Chemical-Structures/chemical-structure-cas-123-11-5.jpg-650.jpg)



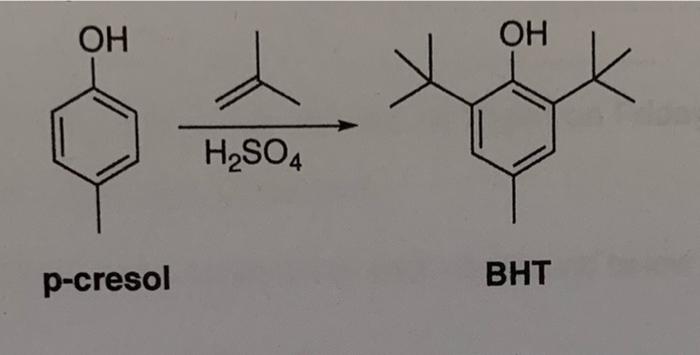


![Propyne []HgSO4 + H2SO4(1%) P Identify the product P. Propyne []HgSO4 + H2SO4(1%) P Identify the product P.](https://dwes9vv9u0550.cloudfront.net/images/1112495/0458b575-c63e-4009-82b5-2bf0590f3e45.jpg)