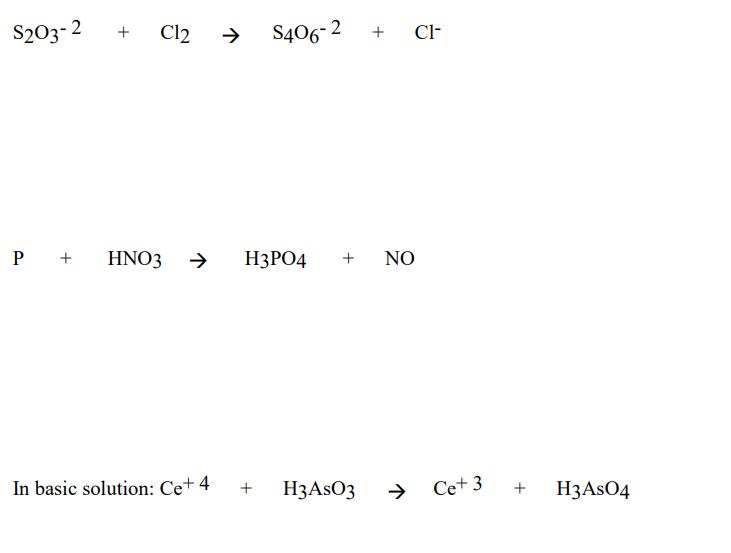
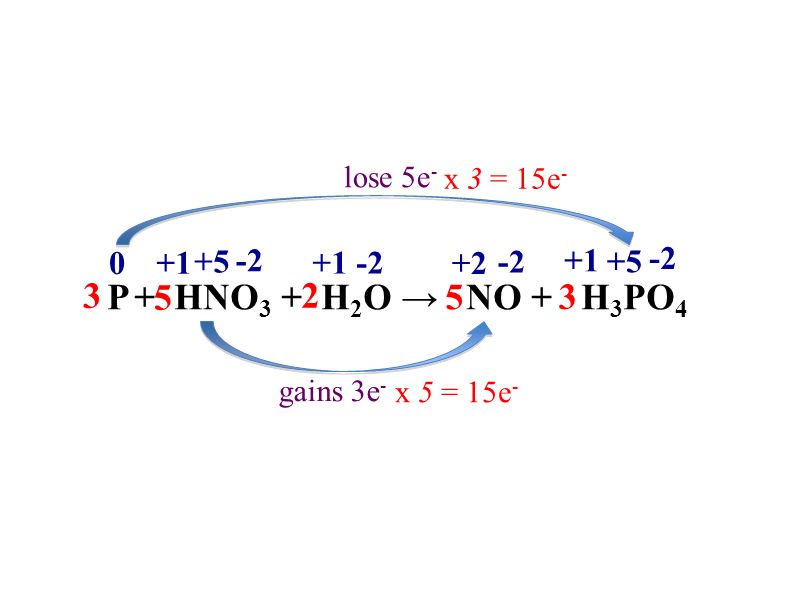
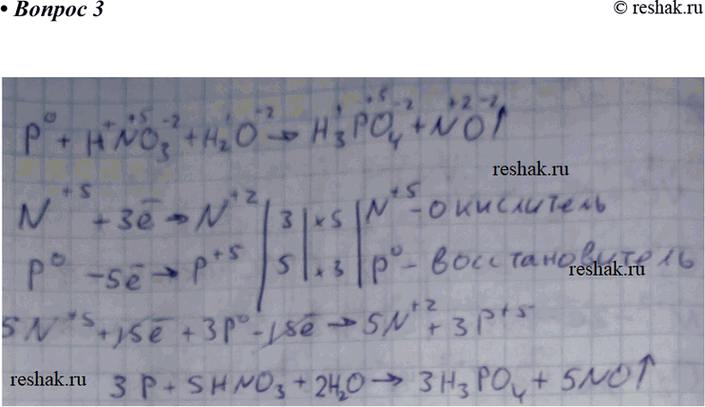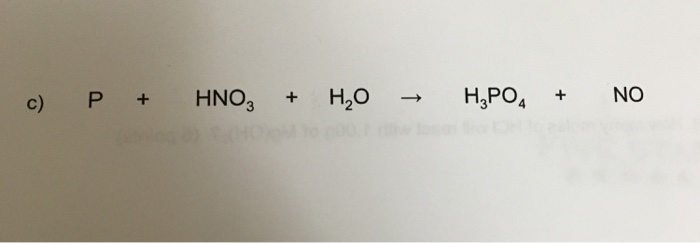Oxidation Number method. P4+HNO3+H2O=H3PO4+NO. Balance the equation by oxidation Number method. - YouTube

a) P HNO3 /P perox ratios derived from box modeling for periods of O 3... | Download Scientific Diagram
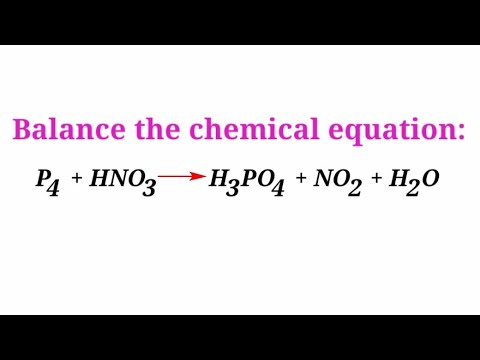
Balance the chemical equation by ion electron and half reaction method. P4+ HNO3=H3PO4+NO2+H2O - YouTube

Algebraic method. P4+HNO3=H3PO4+NO2+H2O balance the equation by a,b,c method. p4+hno3=h3po4+no2+h2o - YouTube

Concentrated nitric acid oxidised phosphorus to phosphoric acid according to the following equation: P+5HNO3(conc.)---> H3PO4 +H2O +5NO2 If 9.3 g of phosphorus was used in the reaction, calculate: I) Number of moles

Which of the following on oxidation with alkaline KMnO4 followed by acidification with dil. HCl gives terephthalic acid?

HNO3/H3PO4–NANO2 mediated oxidation of cellulose — preparation and characterization of bioabsorbable oxidized celluloses in high yields and with different levels of oxidation - ScienceDirect