Tuning the Redox Potentials and Ligand Field Strength of Fe(II) Polypyridines: The Dual π-Donor and π-Acceptor Character of Bi
Rhodium Rainbow: A Colorful Laboratory Experiment Highlighting Ligand Field Effects of Dirhodium Tetraacetate
Give the number of ligand(s) which is/are non-classical ligand an pi donor as well as pi acceptor ligand CO,PH(3), PF(3),C(3)H(5)^(Θ) ,C(5)H(5) Θ .

inorganic chemistry - Why CO is a stronger and more common ligand than N2? - Chemistry Stack Exchange
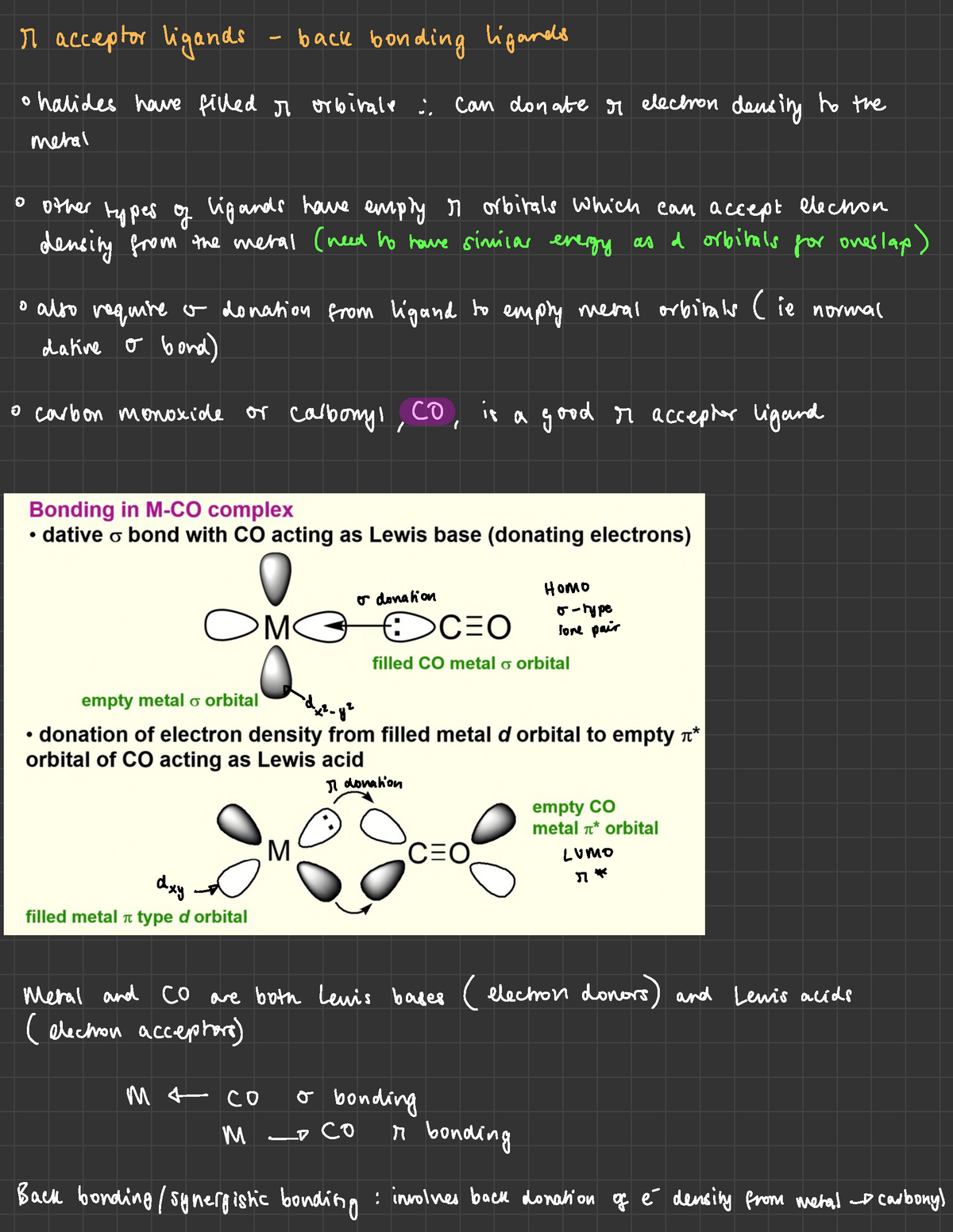
Sigma-donor - Pi-acceptor Ligands - M ####### acceptor ligands back bonding ####### ligands ° - Studocu